UK Biobanking Showcase highlights
The UKCRC Tissue Directory and Coordination Centre (TDCC) held their annual conference UK Biobanking Showcase 2020 between the 12th and 16th October. This year's conference was comprised of webinars and informal meet-ups throughout the week with attendance peaking at over 145 delegates. Here we take a look at some of the Biobanking Showcase highlights of the week and link to the sessions' recordings.
What can we learn from COVID-19?
The week kicked off with session one “What can Biobanking learn from COVID-19?” Engagement Manager Emma Lawrence presented some of the TDCC’s work to coordinate COVID-19 samples earlier this year. The TDCC surveyed 220 UK biobanks in March to find out who could collect and share samples and 139 responded. During the session, 50% of the attendees polled were not aware of these coordination activities, suggesting we need to work harder to communicate our work.
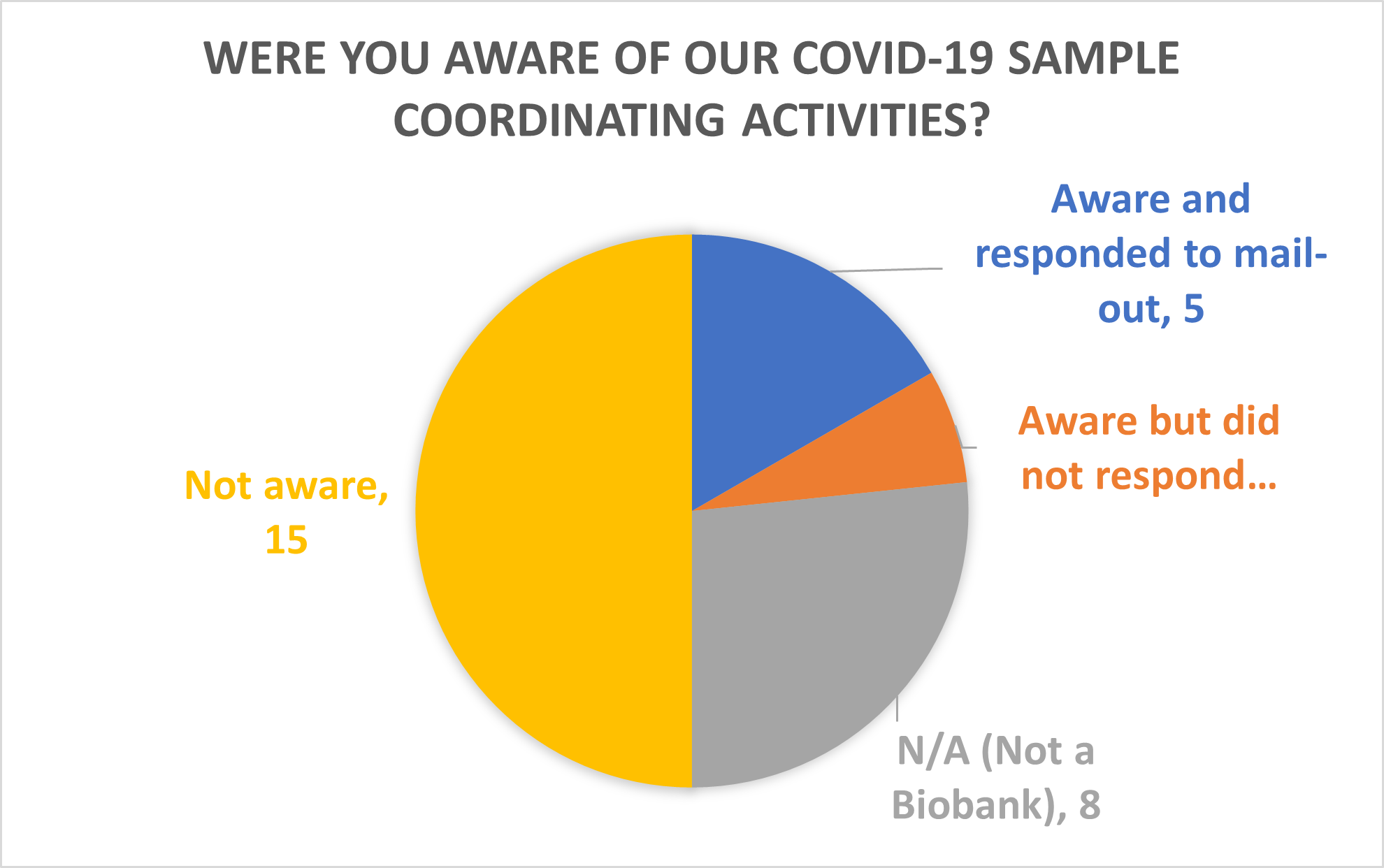
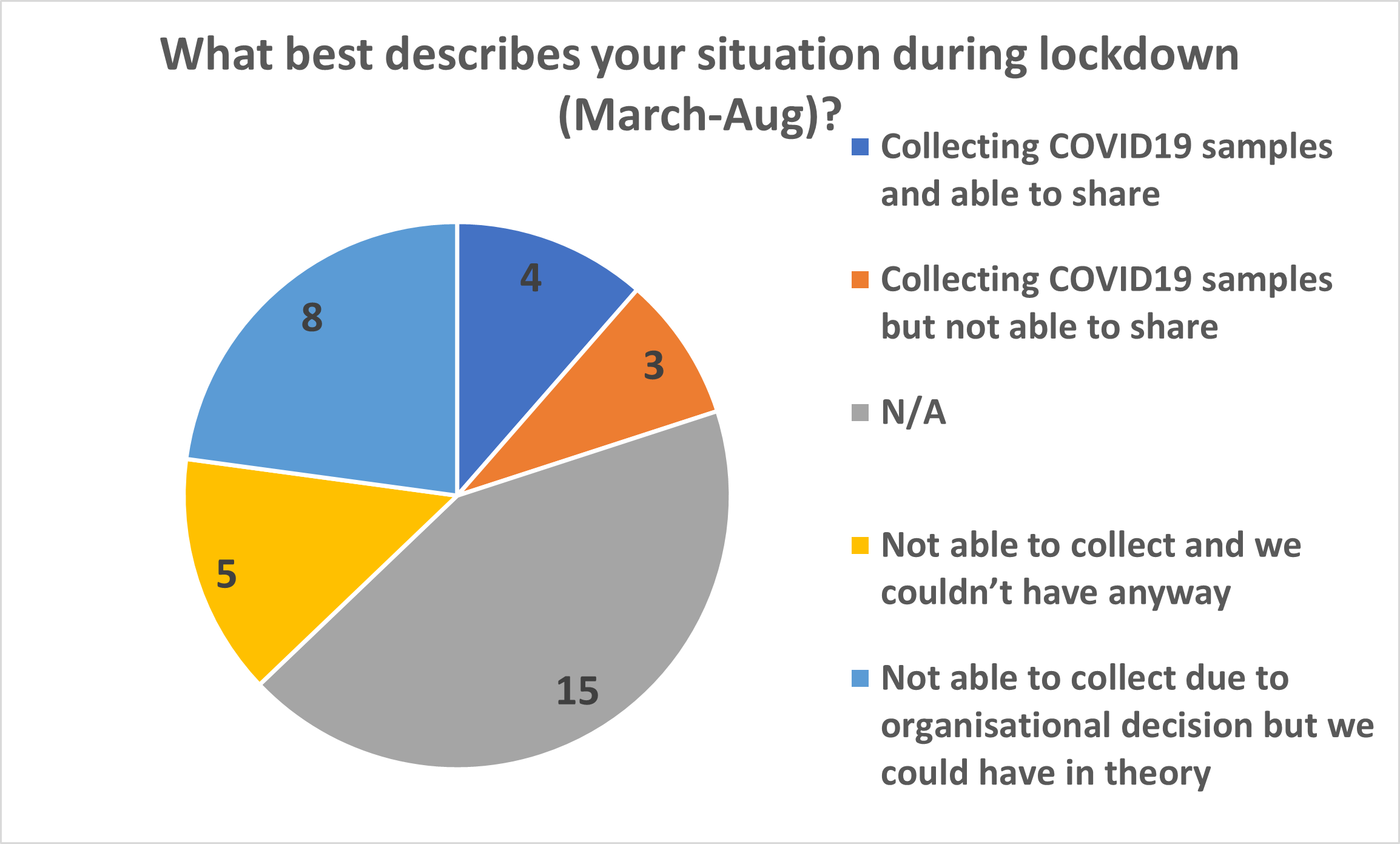
The results of the March survey showed that 80% of Biobanks were closed during the pandemic due to site closures and staff being reallocated or asked to work from home. Twenty-two percent of conference attendees reported they would they have been able to collect COVID-19 samples had been open. Emma’s talk concluded by highlighting the success stories that came from the TDCC’s coordination work: a partnership between Tissue Access for Patient Benefit (TAPB), Cambridge Stem Cell Biobank and Tissue Solutions; and Wales Cancer Bank (WCB) changing the terms in their ethics.
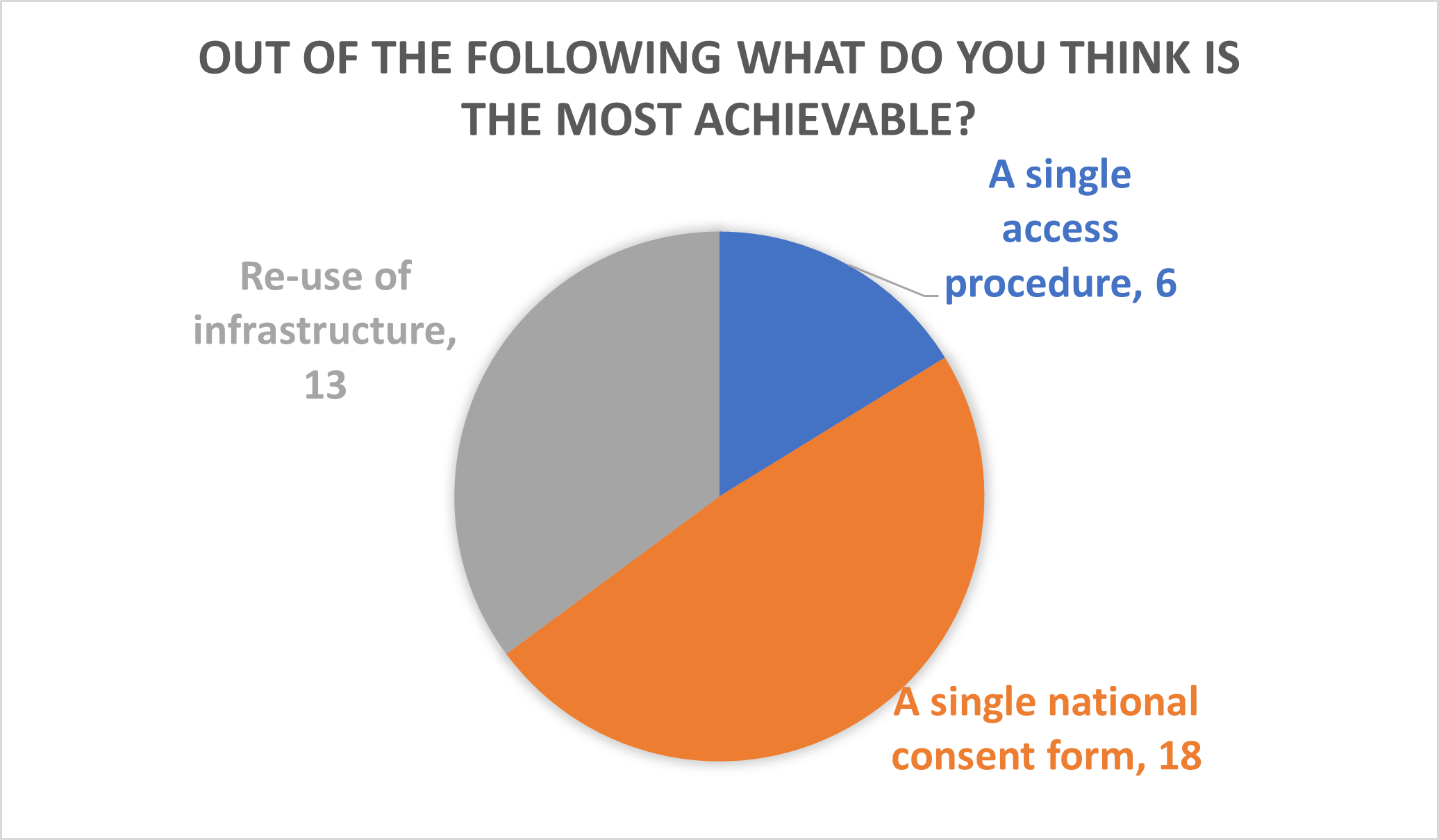
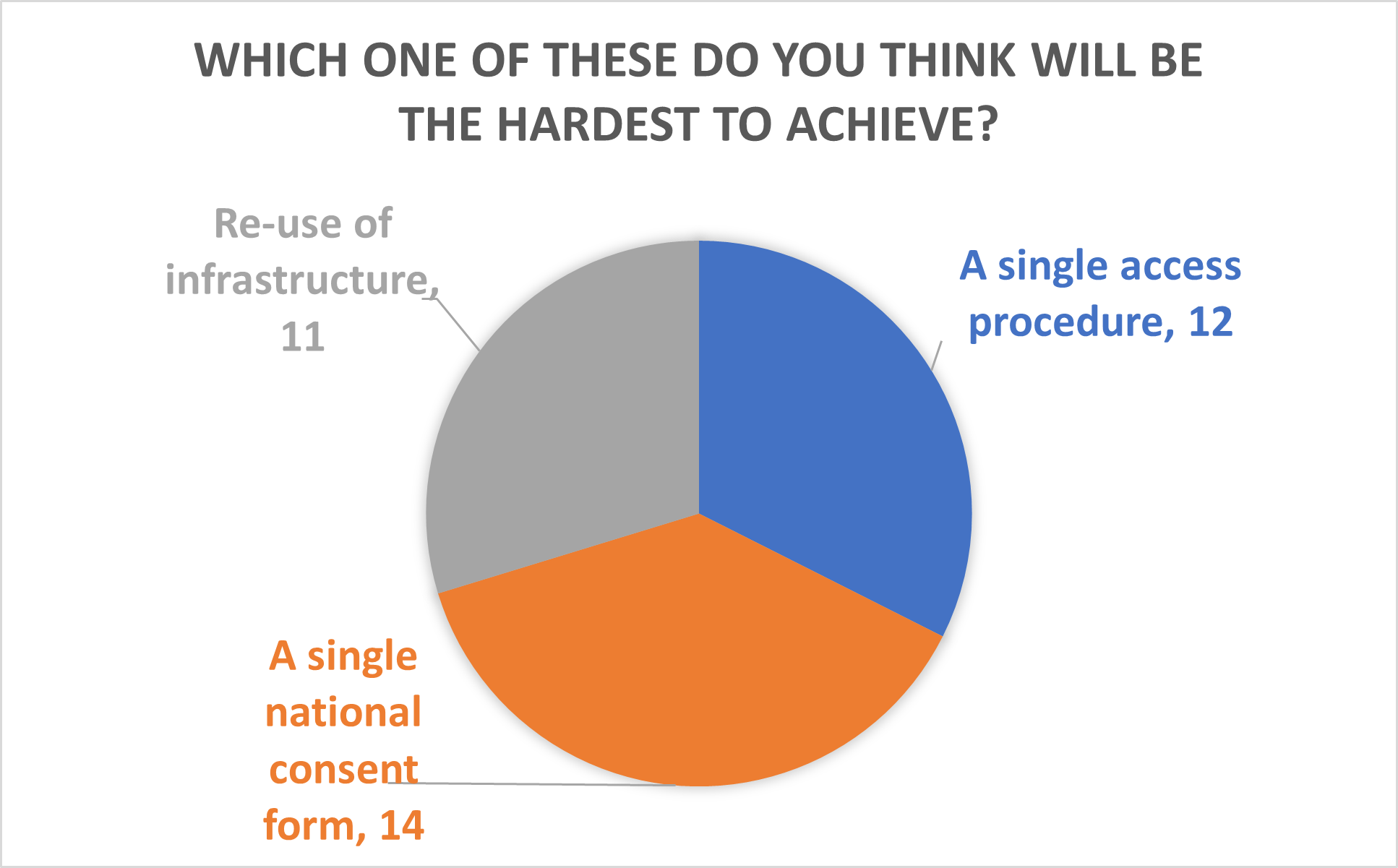
Phil Quinlan, Director of the UKCRC TDCC, finished by talking about what lessons the biobanking community can learn from the COVID-19 pandemic. These included: the need for sample handling facilities at scale and for federated data and sample infrastructure. Phil then proposed solutions, including proactive consenting, re-use of infrastructure and prioritising sample use in a single access procedure. Phil asked which activities attendees thought would be the most achievable and difficult in the session. The answer to both questions was a single national consent form.
Collecting COVID-19 samples
Phil handed over to Amir Gander of TAPB to close the session. Amir started his talk by introducing TAPB which usually collects samples on the basis of demand. TAPB switched to exclusively collecting COVID-19 samples during the pandemic. Amir discussed some of the challenges they faced, such as lack of PPE, the number of patients, the mortality rate and the stressful the working environment. TAPB were able to take samples from approximately 28% of the COVID-19 patients at their hospital despite these challenges. Three-hundred patient samples were shipped in total. Amir then took questions from the audience which included topics such as how to consent critically ill patients. Amir confirmed that they first checked with the clinical team but would not take samples from those without capacity to consent. They also amended their ethics so they could take verbal consent.
Check out the recording of the session to hear more about TAPB’s efforts during the pandemic via the TDCC YouTube Channel.
Sustainability and transparency
The Biobanking Showcase highlights from the second session included speakers giving their perspectives on sustainability. Joseph Fitchett, Medical Director at Mologic, a UK based biotechnology company specialising in rapid diagnostics, kicked off the session. Mologic embarked on the development of a rapid self-administered COVID-19 test at the beginning of the pandemic. However, they felt that samples then became overpriced. Joseph went on to discuss the need for biobanks for epidemics, raising issues such as – what is a fair price? And who should you trust? His challenge accessing COVID-19 samples had a negative impact on Mologic's ability to develop rapid diagnostics. This caused him to question, in his talk, whether the current model is fit-for-purpose.
Learning from animal facilities
Jamie Stock, Finance Manager at the UCL BSU, then discussed how the UCL animal house recovers their costs. He started by introducing the facility and highlighting the similarities in funding between human sample biobanking and animal facilities. HEFCE developed the costing methodology called TRAC with the aim of bringing about cost transparency for funders. The BSU itemises and reviews their costs every three years. He warned that pennies make a big difference to both users and the facility. Jamie concluded by summarising some of the challenges they face, such as a lack of income due to this year’s pandemic.
Business planning for projects
Monica Fletcher, partnerships manager for BREATHE ended the session with a presentation on the BREATHE HDR UK hub. She went on to discuss their journey to being sustainable, referencing the development of a commercial framework which should still serve the public interest. She introduced the basics of business planning, highlighting the need to decide the business and operating model which will then inform the costing model. Monica recommended using business development specialists within universities – who will be able to showcase existing models. She finished by making the comparison between service/cost recovery-based models and outcome-based models, each of which have pros and cons.
Alison Parry Jones, Operations Director (WCB), joined the speakers during the session to continue the discussion on sustainability. Find out more by watching the session online via the TDCC YouTube Channel.
Community, collaboration and consensus
On Wednesday Prof Andy Hall chaired and presented the day's session on the topic of developing common biobanking documentation. Andy began by giving an insight into the REC approval process, adding that the RECs aim is not to hinder research but make the process better for patients. Andy covered some benefits of using shared documentation, such as being able to translate documentation to be more inclusive to speakers of other languages. Also consensus could improve the quality and the content of patient information, such as including animal use. The session divided attendees in breakout groups to identify common themes found in biobank's documentation and their importance. The attendees pre-submitted the documents. The session closed with Andy reviewing each groups' work.
We will be organising a follow up session to develop further the possibility of common documentation. Please sign up to our newsletter for updates as they develop. You can catch up on Andy’s talk and view the breakout group boards now.
Fair access to samples
The following day Mavis Machirori, Research fellow at the University of Glasgow, opened the session by talking about fair access in biobanking. She stressed the importance of the intentions of people accessing samples being in line with the expectations of sample donors. Mavis discussed defining benefit and how this might look for the different parties. She highlighted the importance of engaging with donors, so that they know residual samples may be used in research. Mavis also stressed the importance of dialogue and forming social contracts in the exchanges. She finally referenced the need for guidance on what is allowed, transparency about processes, and also actionable penalties for those who breech the rules.
The session divided attendees into breakout groups to redesign biobanking from the point of view of the donor, funder, academic researcher, commercial researcher, funder and biobanks. Amanda Gibbon, Chair of the UKCRC TDCC Steering Committee and of the day's session, then discussed with Mavis the groups' redesigned systems. Check out the group work and the recording of the session now.
Getting found online
Friday Amanda O’Brien, marketing consultant and blogger, ended the week with her presentation on improving one’s online presence. Amanda explained how search engines work and how to improve your website/page findability. She gave many helpful tips such as keeping your pages on a website with a higher domain authority such as your university or the UKCRC Tissue Directory. She then explained how to optimise the content of your webpage using keywords. Amanda finished by giving an overview of how to use social media. You can watch the whole presentation for more tips on improving your online presence on the TDCC YouTube Channel.
2020 Biobank of the Year
We ended the day and conference by presenting UK Biobank of the Year award to Wales Cancer Bank! Congratulations to them and to Tommy’s National Reproductive Health Biobank for getting an honourable mention from the panel. Many thanks to all those who entered, please take a look at this year’s entries.
Thanks again to everyone who participated in this year’s virtual event and we hope to see you in person next year!

